Control Substance Inventory Michigan PDF Form
In Michigan, the handling of controlled substances is subject to meticulous regulation, underpinned by procedures designed to ensure accountability and compliance with state and federal laws. Among these procedures, the Michigan State University Annual Controlled Substance Inventory Form plays a critical role. This form, mandated for completion between April 1 and June 30 each year, is an essential tool for entities registered to handle such substances. It requires a comprehensive listing of all controlled substances at each registered location, including details about DEA registration numbers, the specific substances on hand, and their quantities, concentrations, and forms, such as vials or syringes. Notably, substances classified under DEA Schedules I and II are to be distinctly listed or documented on a separate form, underscoring the heightened regulatory scrutiny they attract. Further, the inventory process mandates the involvement of at least two individuals - the person performing the inventory and a witness - both of whom must affirm the accuracy of the information provided through their signatures. Once completed, the form must be dispatched to the State of Michigan, Bureau of Health Professions- Health and Regulatory Division, while a signed and complete copy is retained at the licensed premises. This document also satisfies the requirements for the biennial inventory mandated by the Drug Enforcement Administration (DEA), illustrating its dual utility in regulatory compliance. The careful adherence to this process not only aligns with legal obligations but also promotes a framework of diligence and accountability in the management of controlled substances.
Preview - Control Substance Inventory Michigan Form
Michigan State University
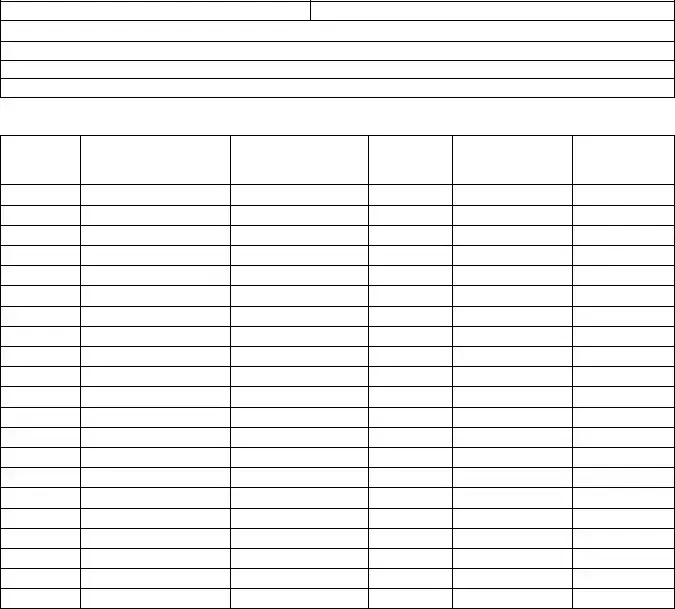
Annual Controlled Substance Inventory Form
Inventory must be performed between April 1 and June 30 of each year. A separate annual inventory is required for each registered location. Mail to: State of Michigan, Bureau of Health Professions‐ Health and Regulatory Division, Annual Inventory, 6546 Mercantile Way, Suite 2, P.O. Box 30454, Lansing, MI 48909. Retain a signed and completed copy of this form at the licensed location. The completed form can serve as the biennial inventory required by the DEA.
Date:
Start of day |
End of day |
MI Licensee/DEA Registrant Name:
MI Licensee/DEA Registrant Address:
DEA Registration #:
State of MI Controlled Substance ID #:
DEA Schedule*
Controlled Substance
Container Unit Type (Vial, syringe, patch, etc.)
Container Quantity
Container Volume
Concentration
*Schedule I and II controlled substances must be separated from all other substances or places on a separate form.
Inventory performed by: _________________________________ |
____________________________________________ |
Print Name |
Signature |
Inventory witnessed by: _________________________________ |
____________________________________________ |
Print Name |
Signature |
|
Page: ___ of_ __ |
Form Characteristics
| Fact | Detail |
|---|---|
| Annual Inventory Timeframe | The inventory must be performed between April 1 and June 30 each year. |
| Requirement for Separate Inventories | A separate annual inventory is required for each registered location. |
| Submission Address | Forms are to be mailed to the State of Michigan, Bureau of Health Professions‐ Health and Regulatory Division, Annual Inventory, 6546 Mercantile Way, Suite 2, P.O. Box 30454, Lansing, MI 48909. |
| Record Retention | A signed and completed copy of the form must be retained at the licensed location. |
| Biennial Inventory Compatibility | The completed form can serve as the biennial inventory required by the DEA. |
| Schedule I and II Separation Requirement | Schedule I and II controlled substances must be separated from all other substances or placed on a separate form. |
Guidelines on Utilizing Control Substance Inventory Michigan
Completing the Control Substance Inventory form for the state of Michigan is a necessary procedure for entities registered to handle controlled substances within the state. This form, part of a regulatory compliance process, ensures that the handling, storage, and usage of such substances are accurately recorded and monitored. It aids in preventing misuse and theft by maintaining a transparent record that the state can audit. The process of filling out the form is meticulous and must be carried out with attention to detail between April 1 and June 30 annually. Each location registered must submit a separate inventory form. Here’s a step-by-step guide on how to properly complete the form:
- Identify the Inventory Timing: Indicate whether the inventory is being conducted at the start or the end of the day by checking the appropriate box.
- Provide Registrant Information: Fill in the name and address associated with the MI Licensee/DEA Registrant. Ensure that the details match those on record with the state.
- Record License and Registration Numbers: Enter the DEA Registration number and the State of MI Controlled Substance ID number in the designated spaces.
- List Controlled Substances: For each controlled substance, specify the DEA Schedule. Remember, Schedule I and II substances must be either listed separately or placed on a separate form to distinguish them clearly from other substances.
- Detail Substance Information: For each entry, specify the type of container (e.g., vial, syringe, patch), the quantity of containers, the volume per container, and the concentration of the controlled substance. Each line should detail a specific controlled substance and its associated data.
- Sign and Date the Inventory: After completing the inventory, the person performing the inventory must print their name and sign the form. This acts as a declaration that the information provided is accurate and complete.
- Obtain a Witness Signature: The inventory process requires a witness. Have another individual print their name and sign the form as a confirmation of the inventory's completion. This step ensures additional accountability.
- Record Pagination: If your inventory requires more than one page, be sure to number each page accurately (e.g., page 1 of 2, page 2 of 2) to ensure the entire inventory is accounted for and can be easily referenced.
- Mail the Completed Form: Send the original completed form to the State of Michigan at the address provided at the top of the form. Keep a signed and completed copy at the licensed location for your records.
Once the form is duly filled and dispatched to the Bureau of Health Professions - Health and Regulatory Division, it serves a dual purpose. It not only meets the state’s annual requirement but also aligns with the biennial inventory requirements of the DEA, thereby streamlining compliance efforts across different regulatory layers. Ensure that the completed form is accurate and submitted within the timeline specified by the state to maintain compliance and avoid potential legal complications.
Crucial Points on This Form
What is the purpose of the Michigan State University Annual Controlled Substance Inventory Form?
This form is designed to maintain accurate records of controlled substances as required by law. It ensures compliance with both state and federal regulations, facilitating the monitoring and control of these substances to prevent misuse and illegal distribution. The completion of this inventory supports the efforts in combating drug abuse and diversion.
When should the inventory be performed and submitted?
The inventory must be performed annually between April 1 and June 30. After completion, the form should promptly be mailed to the State of Michigan, Bureau of Health Professions - Health and Regulatory Division, at the provided address. It's critical to meet this timeframe to ensure compliance with regulatory requirements.
Is a separate inventory required for each registered location?
Yes, if you have controlled substances at more than one registered location, a separate annual inventory must be completed for each location. This approach helps in maintaining specific, accurate records for each location, which is essential for regulatory compliance and effective monitoring.
Where should the completed inventory form be sent?
The completed inventory form should be mailed to the State of Michigan, Bureau of Health Professions - Health and Regulatory Division, at the address: Annual Inventory, 6546 Mercantile Way, Suite 2, P.O. Box 30454, Lansing, MI 48909. This ensures that your inventory records are properly filed with the state authorities.
What should be done with a copy of the completed form?
A signed and completed copy of the form must be retained at the licensed location. Keeping this record ensures your facility has documented evidence of compliance with inventory requirements, available for review in the case of an inspection by regulatory authorities.
Can the form serve as the biennial inventory required by the DEA?
Yes, the completed form can also fulfill the requirements for the biennial inventory necessary under Drug Enforcement Administration (DEA) regulations, as long as it is properly filled out and retained. This dual-purpose nature streamlines the process, reducing paperwork and ensuring adherence to both state and federal guidelines.
What information is required on the form for each controlled substance?
The form requires detailed information for each controlled substance, including:
- DEA Schedule
- Name of the controlled substance
- Container type (e.g., vial, syringe, patch)
- Container quantity
- Container volume
- Concentration of the substance
How should Schedule I and II controlled substances be recorded?
Schedule I and II controlled substances must be separated from all other substances or placed on a separate form. This requirement reflects the higher potential for abuse and stricter regulatory controls associated with these substances, ensuring they receive the appropriate level of scrutiny.
Who must sign and witness the inventory?
The inventory must be both performed and witnessed by authorized individuals. These individual's printed names and signatures must be included on the form. This dual verification process adds an additional layer of oversight, aimed at enhancing the accuracy and reliability of the inventory records.
Common mistakes
Filling out the Control Substance Inventory for Michigan is a critical task that requires attention to detail. Unfortunately, people often make several common mistakes during this process. One frequent error is not conducting inventories within the specified timeframe, which is between April 1 and June 30 each year. This detail is crucial because inventories completed outside this window may not comply with state requirements, leading to potential legal and administrative complications.
Another mistake involves not providing a separate inventory for each registered location. The Michigan State University Annual Controlled Substance Inventory Form clearly states that a distinct inventory must exist for every location. Not adhering to this requirement can cause significant confusion and inaccuracies in tracking controlled substances, potentially resulting in serious legal issues.
People also often overlook the importance of correctly identifying the DEA Schedule of the controlled substances. Each substance must be correctly classified according to its DEA Schedule, and most notably, Schedule I and II substances must be separated from all others or listed on a separate form. Misclassification can lead to non-compliance with both state and federal regulations, posing risks of fines or sanctions.
Incorrectly recording the controlled substance details, such as container type, quantity, volume, and concentration, is another common error. These details are vital for accurate inventory tracking and management. Mistakes in this area can lead to discrepancies in inventory records, which are problematic during audits or inspections.
Additionally, the requirement for the inventory to be signed and witnessed is sometimes overlooked. The form mandates that the inventory be signed by the person conducting it and witnessed by another individual. This step is essential for the validation of the inventory's accuracy and integrity. Failure to have the form properly signed and witnessed undermines its legitimacy and may lead to compliance issues.
Finally, another error is failing to retain a signed and completed copy of the inventory at the licensed location, as stipulated by the form instructions. This retention is necessary not only for compliance with state requirements but also because the completed form can serve as the biennial inventory required by the DEA. Not having this crucial document on hand can result in complications during regulatory reviews or audits.
Documents used along the form
Managing controlled substances requires meticulous documentation to ensure compliance with regulations. The Control Substance Inventory Michigan Form is a crucial tool in this process. However, to maintain proper records and comply with all regulatory requirements, several other forms and documents are commonly used alongside this form. Understanding each of these documents can help individuals and institutions streamline their record-keeping and avoid regulatory pitfalls.
- DEA Form 222: This form is used for ordering Schedule I and II controlled substances. It serves as an official order form and is meticulously tracked to prevent the misuse and diversion of these highly regulated substances.
- Biennial Inventory Report: While the Control Substance Inventory Michigan Form can serve as the biennial inventory required by the DEA, entities often prepare a separate, comprehensive Biennial Inventory Report. This report provides a detailed account of all controlled substances on hand at a facility, documenting their quantity and strength, to comply with DEA regulations that mandate a complete inventory every two years.
- Record of Receipts: This log tracks all controlled substances received at a facility. It details the date of receipt, the quantity and type of substance received, the name of the supplier, and the DEA registration number of the supplier. Keeping accurate and up-to-date records of receipts is essential for managing inventories and ensuring accountability.
- Dispensing/Usage Log: This document records each instance a controlled substance is dispensed or used within a facility. It includes the date, the quantity dispensed, the name of the recipient or patient, and the purpose of dispensation or use. This log helps maintain a clear record of how substances are being utilized and is vital for auditing and compliance purposes.
- DEA Form 106: In the event of theft or significant loss of controlled substances, DEA Form 106 must be completed and submitted to the DEA. This form documents the circumstances of the loss, the quantity and type of substances lost, and the measures taken to prevent future losses. Prompt reporting is critical to help mitigate the impact of such incidents and comply with legal requirements.
Together, these documents form a comprehensive framework for managing controlled substances responsibly and in compliance with both state and federal regulations. By carefully maintaining these records, facilities can ensure they meet all legal requirements, safeguard against the diversion of controlled substances, and provide transparency in their handling and usage.
Similar forms
The Pharmacy Inventory Audit Log: This document is similar to the Michigan Controlled Substance Inventory Form as it meticulously tracks the quantities and details of medications within a pharmacy. Both forms are tools for maintaining accurate records of controlled substances, ensuring compliance with regulatory standards. They require the name and address of the facility, details about the drugs (including drug schedule, volume, and quantity), and verification by authorized personnel.
The Hazardous Materials Inventory Statement (HMIS): While focusing on a different type of inventory, the HMIS shares similarities with the Michigan form in terms of its purpose for regulatory compliance and safety. Both documents require detailed listings of substances, their amounts, and locations. Although the HMIS is more focused on safety data sheets and hazard classifications, both forms are critical for maintaining safety and regulatory compliance within their respective fields.
The Asset Inventory Record: Employed by businesses to track physical and digital assets, this document parallels the Michigan Controlled Substance Inventory Form in its detailed recording of specific items, their location, and quantity. Despite focusing on broader asset categories, both forms play crucial roles in organizational accountability and regulatory compliance, ensuring accurate record-keeping for auditing and reporting purposes.
The Food Inventory Control Sheet: Used in the food service industry to track stock levels, purchase dates, and quantities of ingredients, this document mirrors the Michigan form's objectives in inventory management. Both aim to prevent shortages or overstock through meticulous record-keeping, offering a snapshot of available resources at any given time to ensure smooth operations and compliance with industry regulations.
Dos and Don'ts
Filling out the Control Substance Inventory form for Michigan is a critical task that ensures compliance with both state and federal regulations. When completing this form, certain practices should be followed to ensure accuracy and legality, whereas others should be avoided to prevent potential issues. Here are some guidelines to consider:
Things You Should Do
- Perform and submit the inventory within the specific timeframe: Ensure the inventory is conducted and mailed between April 1 and June 30 of each year.
- Use a separate form for each registered location: If controlled substances are managed at multiple locations, complete and submit an individual inventory form for each one.
- Sign and retain a copy: After filling out the form, the person responsible should sign it. Keep a signed copy at the licensed location for legal and verification purposes.
- Record the exact quantities: Precisely document the quantity, volume, and concentration of each controlled substance to ensure accuracy.
Things You Shouldn't Do
- Don't mix schedules: Separate Schedule I and II controlled substances from others—or better yet, use a separate form for them as required.
- Avoid inaccuracies: Do not estimate or round off the quantities of substances. Precise measurements are crucial for legal and operational reasons.
- Don’t forget witness details: Failing to have the inventory witnessed and omitting the witness's printed name and signature could invalidate the process.
- Never submit incomplete forms: Ensure every field relevant to your inventory is filled out. Leaving sections blank can lead to regulatory noncompliance.
By adhering to these do's and don'ts, the process of completing the Michigan Controlled Substance Inventory form can be conducted smoothly and effectively, ensuring compliance with regulatory requirements and contributing to the responsible management of controlled substances.
Misconceptions
Many people have misconceptions about the Controlled Substance Inventory in Michigan, particularly regarding the Michigan State University Annual Controlled Substance Inventory Form. Understanding these misconceptions can help in correctly managing controlled substances within the state.
Misconception 1: The inventory is only for Michigan State University. Some might think that this inventory form is exclusively for use by Michigan State University due to its title. However, it is a requirement for all entities in Michigan that manage controlled substances, not just for the university. The form's title might simply refer to the format adopted or provided by MSU as a guideline.
Misconception 2: It is optional to complete. There's a misconception that completing the inventory form is optional. This is false. The State of Michigan mandates the annual inventory for each registered location, ensuring adherence to both state and federal regulations concerning controlled substances.
Misconception 3: The inventory does not need to be retained. Some might incorrectly assume that once the inventory form is mailed to the State of Michigan, Bureau of Health Professions, there is no need to retain a copy at the licensed location. Contrary to this belief, a signed and completed copy of the form must be kept at the licensed location as part of regulatory compliance.
Misconception 4: The inventory form covers multiple locations. There is a belief that one form can be used for multiple registered locations. Each registered location must complete a separate annual inventory, ensuring specific and accurate reporting for each site.
Misconception 5: All controlled substances can be listed together regardless of their DEA schedule. The inventory form clearly requires that Schedule I and II controlled substances be separated from other substances or listed on a separate form. This important detail helps in maintaining stringent control and oversight over substances with a high potential for abuse.
Misconception 6: The inventory timing is flexible. Some may believe that the inventory can be performed at any time of the year. However, the inventory must be conducted annually between April 1 and June 30, ensuring a standardized period across all entities for inventory assessment.
Clearing up these misconceptions is crucial for compliance with Michigan's controlled substances management and regulatory requirements. Diligence in understanding and adhering to these guidelines helps prevent legal issues and promotes safe handling of controlled substances.
Key takeaways
When completing the Control Substance Inventory Michigan form, there are several critical points professionals need to keep in mind. Ensuring accuracy and compliance with regulatory standards not only helps in maintaining the integrity of the inventory process but also adheres to state and federal laws governing controlled substances.
Inventory Timing: The inventory must be carried out annually between April 1 and June 30. This specific timeframe is crucial for compliance and should be adhered to in order to avoid potential legal repercussions.
Location-specific Inventory: For every registered location, a separate inventory is required. This means if controlled substances are stored or utilized in multiple locations, each location’s inventory must be documented independently.
Sending the Completed Form: The completed form should be mailed to the State of Michigan, Bureau of Health Professions - Health and Regulatory Division. Keeping a careful record of the date sent and ensuring it reaches the intended destination is important for compliance.
Record Retention: A signed and completed copy of the inventory form must be retained at the licensed location. This serves as a critical document for both state compliance and in serving as the biennial inventory required by the DEA.
Distinction of Controlled Substances: Schedule I and II controlled substances must be listed separately from other substances or on a separate form entirely. This distinction is fundamental in maintaining the clarity and accuracy of the inventory record, ensuring that the most tightly controlled substances are adequately monitored.
Witness Requirement: The inventory process must be both performed and witnessed by individuals who then must print and sign their names on the form. The inclusion of a witness serves as an additional layer of verification, adding to the integrity of the inventory process.
Adhering to these guidelines is essential for ensuring that the Control Substance Inventory Michigan form is filled out correctly and in compliance with applicable laws. This not only helps in the precise tracking and monitoring of controlled substances but also aids in safeguarding against potential misuse or diversion, thereby contributing to the broader objective of public health and safety.
Popular PDF Templates
Michigan Form 151 - Reflects a business’s commitment to maintaining tax compliance through authorized representatives.
5 Criteria for Involuntary Admission Michigan - Filing this form in the circuit court family division requires specific identification of the court name and county, underscoring its legal significance.